A high-tech analysis of an amalgam filling that is a couple of decades old revealed that up to 95% of its surface mercury content was missing, according to a team of researchers from the University of Saskatchewan (Chemical Research in Toxicology, October 20, 2009, epub ahead of print).
But the investigators also found the remainder was sequestered in a stable form that is less toxic.
In a first step toward quantifying the mercury exposure from dental amalgam, the researchers placed several teeth with fresh dental amalgams and a tooth with an amalgam that was at least two decades old into a synchrotron, a very large machine that examines the physical and chemical properties of substances at an extremely high level of resolution.
They found that the surface of the older amalgam had only 5% of the mercury content of the new amalgam. The 5% of the mercury that remained on the surface of the older amalgam was in the form of metacinnabar, an ore of mercury that is virtually insoluble in water and very resistant to attack by acids and bases.
— Graham George, University
of Saskatchewan
“The dental amalgam on the surface of an old tooth filling may have lost as much as 95% of its mercury, but what’s left is in a form that is unlikely to be toxic in the body,” said Graham George, B.Sc., D.Phil., the study's principal investigator and the Canada Research Chair in X-ray Absorption Spectroscopy at the University of Saskatchewan. "The fact that metaccinabar is so insoluble in water means it's essentially biounavailable to act as a toxin. But we don't know what's happened to the other 95% of the mercury."
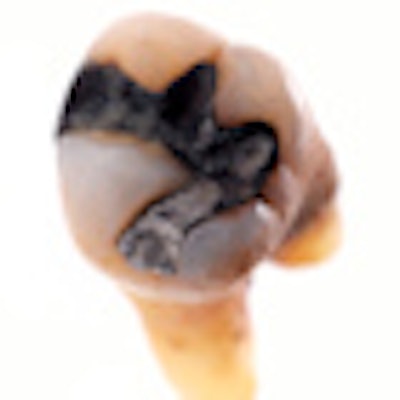
Surface amalgam
The new mercury amalgam was prepared using a standard commercial preparation. The drilling, installation of the filling and burnishing were performed exactly as if they were being done in any dentist's office, using a melamine replacement tooth. The other tooth -- a mandibular second molar with a mesio-occlusal restoration -- was obtained from the University of Saskatchewan Dental Clinic's tooth bank. The filling had a dark patina, and the researchers estimated it had been in the person's mouth for at least 20 years before it was extracted.
The tooth from the tooth bank was carefully washed with distilled water and allowed to dry. It was then mounted on a specially designed electron detector and placed in the synchrotron. The teeth with the fresh amalgams were also mounted on electron detectors and placed in the synchrotron, as was a frozen sample of pure metallic mercury.
"With the synchrotron we were looking at the outer 20 to 50 Angstroms of the surface of the amalgams," explained Dr. George. "That is the chemical surface with which saliva would come into contact; any processes that interact with the teeth would happen in that outer layer."
The results of the synchrotron analysis showed that the electron yield edge jump of the older amalgam filling was as little as 5% of that of the fresh fillings. The researchers interpreted this as meaning that as little as 5% of the mercury content was left in the surface layer of the amalgam.
Where did it go?
However, they noted that there may have been other reasons for the reduction in the electron yield jump edge; hence, the 5% is a lower boundary and may well be higher. The mercury that remained had the electron signature of metaccinabar, a mercuric sulfide compound.
The team speculated that the metacinnabar may have been formed after the person from whom the tooth had been taken had ingested quantities of sulfides in foodstuffs such as coffee, onion, or garlic. They also hypothesized that the metacinnabar may be a result of bacterial action. The researchers intend to verify which, if any, of these processes lead to the creation of metacinnabar in amalgams.
"Whatever the origin [of the metacinnabar], due to [its] biounavailability … loss of particulates from the outer surface of the dental restoratives by teeth grinding or by polishing during dental cleaning is unlikely to cause any toxic effects [once the metacinnabar is formed]," the authors concluded.
But the question remains: what happens to the rest of the mercury once it leaves the amalgam? The team cautions that due to the significant mercury loss over time, human exposure to mercury lost from fillings is “still of concern” and that further research is needed to determine when, how and in what form mercury is lost from fillings.
"We know some of it is lost as vapor, but what happens with mercury when, for example, it's leached into the saliva?" asked Dr. George. "That's what I think is exciting -- we can follow these processes using the synchrotron."