Biolux Research's OrthoPulse has received 510(k) marketing clearance from the U.S. Food and Drug Administration (FDA), the company announced.
"We are thrilled to announce that OrthoPulse has received FDA clearance and that we can begin to market the product in the United States," stated Kevin Strange, president and CEO of Biolux, in a press release. "The impressive clinical results and experience from our key evaluators and existing customers has been every encouraging; with significant acceleration of orthodontic treatment, we envision that OrthoPulse will be a game changer in the world of orthodontics."
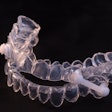
OrthoPulse is a class II medical device that uses low-level light therapy to accelerate orthodontics time. It has already received regulatory approvals in the European Union, Canada, and Australia and New Zealand.