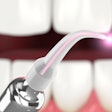
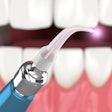
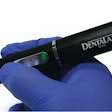
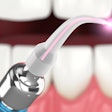
The U.S. FDA has granted 510(k) marketing clearance to Biolase Technology's ezlaze diode laser system for therapeutic medical and dental applications, including temporary pain relief, the company announced.
This makes the ezlaze the only dental diode laser system that has FDA clearance for soft-tissue procedures, teeth whitening, and pain relief, noted Biolase CEO David Mulder in a press release.
Dara Rosenberg, D.D.S., director of the department of dentistry at St. Barnabas Hospital in New York City, has performed multiple pilot studies with low-level laser therapy using a Biolase diode laser for myofacial pain and temporomandibular joint dysfunction on patients who have few treatment options left, Biolase noted.
"Outcomes have been very positive over the years," Dr. Rosenberg said in the release. "These chronic conditions can now be managed noninvasively and nonpharmacologically. Our clinical experience with the laser has shown improvement in both pain relief and return to function. It has enabled patients who have been on medication for extended periods of time to reduce and eliminate the medications and avoid surgical intervention. Because it is noninvasive, more practitioners should expand its use for the benefit of all patients."
The FDA has given clearance for ezlase for the following indications in both dental and medical applications: temporary relief of minor muscle and joint pain and stiffness, minor arthritis pain, muscle spasm, minor sprains and strains, and minor muscular back pain; temporary increase in local blood circulation; and temporary muscle relaxation.
Copyright © 2009 DrBicuspid.com