Navidea Biopharmaceuticals announced that the European Commission has granted marketing authorization for its Lymphoseek 250-mcg kit for radiopharmaceutical preparation.
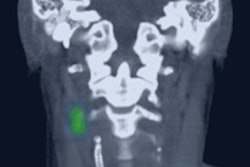
Lymphoseek (technetium-99m tilmanocept) is now approved in the European Union (EU) for use in imaging and intraoperative detection of sentinel lymph nodes draining a primary tumor in adult patients with breast cancer, melanoma, or localized squamous cell carcinoma of the oral cavity.
Navidea CEO Rick Gonzalez stated in a press release that this was an important milestone.
"This is yet another important milestone achieved by Navidea in our global commercialization of Lymphoseek and reaffirms our commitment to improving the lives of oncology patients worldwide," Gonzalez said. "We look forward to making Lymphoseek available throughout Europe with initial launches into certain major markets planned for later in 2015."
Dr. John Buscombe, a physician in nuclear medicine at Addenbrooke's Hospital in Cambridge, U.K., said in the same release that Lymphoseek can be a game changer.
"Lymphoseek can be a game changer in allowing more patients across Europe access to this technology, which allows their cancer to be accurately staged without disfiguring and disabling surgery," Dr. Buscombe said. "The availability and convenience of a receptor-targeted imaging agent such as Lymphoseek, the first synthetic and biotargeted agent for use in sentinel node localization, provides both the required diagnostic accuracy and reliability that enables a surgeon planning sentinel node biopsy to be able to operate without the concerns raised with timing from imaging to surgery found with alternate methods."
Lymphoseek is approved in the U.S. for use in lymphatic mapping to locate lymph nodes draining a primary tumor site in patients with solid tumors for which this procedure is a component of intraoperative management and for guiding sentinel lymph node biopsy using a handheld gamma counter in patients with node-negative squamous cell carcinoma of the oral cavity, breast cancer, or melanoma.