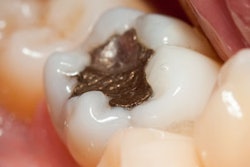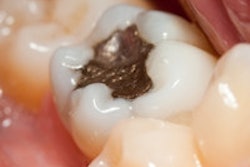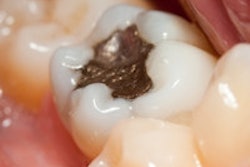
In a final opinion on dental amalgam, a European Commission scientific committee concluded that alternative materials to dental amalgam should be the first choice for certain restorations, such as those in pregnant patients and primary teeth.
The updated opinion by the Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR) updates the committee's previous one published in 2008. It assessed the safety and effectiveness of dental amalgam and possible alternatives, such as resin-based composites, glass ionomer cements, ceramics, and gold alloys, by evaluating the scientific evidence on the potential association of amalgam and its alternatives with allergies, neurological disorders, or other adverse health effects.
This opinion contrasts with the January 2015 U.S. Food and Drug Administration (FDA) update of its consumer advisory on dental amalgam, which did not change the agency's position on amalgam fillings and concluded that such fillings are safe for adults and children ages 6 and older.
Individual patient characteristics
The Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR) of the European Commission wrote that, while "current evidence does not preclude the use of either amalgam or alternative materials in restorative treatment," individual patient characteristics should determine the material used in treatment.
The following patient characteristics should be considered:
- Primary or permanent teeth
- Pregnancy
- The presence of allergies to mercury or other components of restorative materials
- The presence of impaired renal clearance
"Alternative materials have now been in clinical use for well over thirty years, initially in anterior teeth and more recently also for restorations in posterior teeth," the committee wrote. "Existing clinical experience has revealed little evidence of clinically significant adverse events." It noted that the composition of available materials has changed substantially in recent years because of "improved polymerization processes and particular improvement in the adhesive systems and the filler parts." Among the benefits, the committee wrote, is that there is "no evidence that infants or children are at risk of adverse effects arising from the use of alternatives to dental amalgam."
There is a trend toward minimal interventional, adhesive techniques in dentistry, which are "based on adhesion to tooth structure by chemical interaction, and/or micromechanical retention," according to the opinion. At the same time, the committee noted, the quality and durability of alternative materials have improved.
As a general principle, the committee recommended that the relative risks and benefits of any dental treatment should be explained to patients to help them make informed decisions. Also, manufacturers should provide better information concerning the relative risks of dental restorative materials, it added.
An effective material
Dental amalgam is "an effective restorative material for the general population" and has "long been considered the material of choice," the committee noted in its executive summary noted. However, the drawbacks of dental amalgam are well-known, including the exposure to mercury and that it is neither tooth-colored nor can it adhere to remaining tooth tissues. The use of amalgam has been decreasing in recent years, and the alternative tooth-colored filling materials are increasingly used in Europe.
The committee, however, concluded that already-in-place dental amalgam is not considered a health risk for the general population and should not be removed as a preventive measure.
Future research
In the opinion, the committee wrote further research is needed relating to the "evaluation of the potential neurotoxicity of mercury from dental amalgam and the effect of genetic polymorphisms on mercury toxicity." It also recommended that more research be conducted in the development of new alternative materials with a "high degree of biocompatibility" and to expand knowledge of the toxicity profile of alternative dental restorative materials.
More publicly available research data are also needed, and it would be "beneficial for the community in general to be better informed of the recognized benefits and risks," the committee noted.
The opinion corresponds with the intentions of the 2013 Minamata Convention on Mercury to reduce mercury and the general aim to reduce mercury use in the European Union, according to a press release by the European Commission.