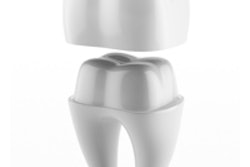
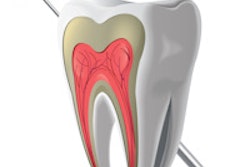
Endodontic technology developer Sonendo has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for the third generation of its Multisonic Ultracleaning system for root canal therapy.
The 510(k) clearance expands the use for the GentleWave system to include anterior and premolar cases, in addition to the current molar indication. The minimally invasive technology is designed to use multiple wavelengths of sound to clean the entire root canal system simultaneously, according to the company.
Sonendo plans to do a phased launch of the system in 2015.