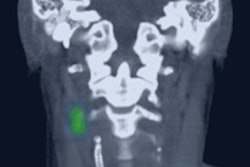
The U.S. Food and Drug Administration (FDA) has approved Navidea Biopharmaceuticals' Lymphoseek radiopharmaceutical to help determine the extent of head and neck cancer (HNC).
Lymphoseek can now be used to help assess the extent to which squamous cell carcinoma has spread in the head and neck, according to the FDA, which approved the agent on June 13. Physicians can use Lymphoseek to guide sentinel lymph-node biopsies, allowing the option of more limited lymph-node surgery in patients with negative sentinel nodes.
For the new indication, Lymphoseek was evaluated in a clinical trial of 85 patients with squamous cell carcinoma of the lip, oral cavity, and skin. Results showed that Lymphoseek-guided sentinel lymph-node biopsy accurately determined if the cancer had spread through the lymphatic system. The most common side effects were pain or irritation at the injection site.
Lymphoseek was previously approved in 2013 for identifying lymph nodes closest to a primary tumor in patients with breast cancer or melanoma, the FDA said.