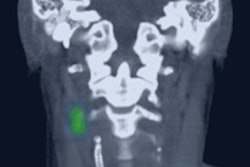
The U.S. Food and Drug Administration (FDA) has approved expanded indications for Navidea Biopharmaceuticals' Lymphoseek radiopharmaceutical agent.
The approval of Navidea's supplemental new drug application covers the use of Lymphoseek -- with or without scintigraphic imaging -- to facilitate preoperative imaging and lymphatic mapping in solid tumors. In addition, Lymphoseek can now be used to guide sentinel lymph node biopsy in patients with breast cancer and melanoma; previously, it was cleared only for guiding these biopsies in patients with clinically node-negative squamous cell carcinoma, according to the company.
The FDA recently granted orphan drug designation to Lymphoseek for use in sentinel lymph node detection in patients with head and neck cancer.
Lymphoseek was approved by the FDA in June to be used to help asses the extent to which squamous cell carcinoma has spread in the head and neck. In 2013, it was approved for identifying lymph nodes closest to a primary tumor in patients with breast cancer or melanoma.