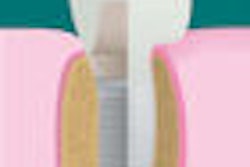
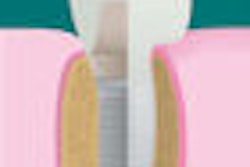
Nasseo's TiArray dental implant system has received 510(k) clearance from the U.S. Food and Drug Administration (FDA).
It is the first commercial application of Nasseo's proprietary surface modification technology, which was initially developed over eight years out of the bioengineering department and the materials science and engineering department at the University of California, San Diego and the Bio-Implant Laboratory at Lund University.
Existing implant surface modifications focus on grit-blasting techniques that have failed to address implant failures and the growing incidence of peri-implantitis, the company noted in a press release.
Nasseo's proprietary implant surface modification technology has the potential to be applied to dental and craniofacial applications that utilize titanium and tantalum as the implant substrate material.