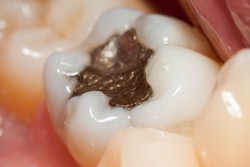
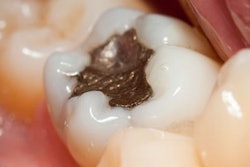
The U.S. Food and Drug Administration (FDA) has updated its consumer advisory on dental amalgam, but it has not changed its position on dental amalgam fillings.
"The FDA has reviewed the best available scientific evidence to determine whether the low levels of mercury vapor associated with dental amalgam fillings are a cause for concern. Based on this evidence, FDA considers dental amalgam fillings safe for adults and children ages 6 and above," states the advisory, updated on January 27. "The weight of credible scientific evidence reviewed by FDA does not establish an association between dental amalgam use and adverse health effects in the general population. Clinical studies in adults and children ages 6 and above have found no link between dental amalgam fillings and health problems."
The FDA advises patients to discuss treatment options with their dentist.
The consumer advisory provides information on the following issues:
- What is dental amalgam?
- What should I know before getting a dental amalgam filling?
- Benefits
- Potential risks
- What is bioaccumulation?
- Is the mercury in dental amalgam the same as the mercury in some types of fish?
- If I am concerned about the mercury in dental amalgam, should I have my fillings removed?
In 2009, the FDA issued a final regulation classifying dental amalgam and its component parts -- elemental mercury and a powder alloy -- as class II devices, even as the agency insists that amalgam poses no health risks for any patient population.
The new rule reclassified mercury from a class I (least risk) device to class II (more risk) device, classifies dental amalgam as a class II device, and designates a special controls guidance document that includes new labeling recommendations for dental amalgam products.
However, while acknowledging that elemental mercury has been associated with adverse health effects at high exposures, FDA officials said "the levels released by dental amalgam fillings are not high enough to cause harm in patients," including pregnant women, developing fetuses, and young children.