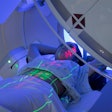
Jason Morgan needs an implant. But like many dental implant candidates, his jaw bone is not strong enough to support it. The 34-year-old man's maxillary molar has been missing so long the bone has resorbed, and his maxillary sinus dropped due to lack of support from the roots.
— Charles Sfeir, D.D.S., Ph.D., director of
the Center for Craniofacial
Regeneration at the University of
Pittsburgh
So his dentist, Ivan Ho, D.D.S., of Rancho Santa Margarita, CA, opted to try something new: a stem cell transplant. On March 23, using a procedure known as bone marrow aspirate concentration, Dr. Ho harvested and transplanted stem cells from Morgan's hip bone marrow into his jawbone.
"This minimally invasive procedure is a long-term alternative to existing tooth replacement options such as dentures, bridges, and even traditional bone grafting procedures," Dr. Ho said. "The transplantation of the patient's own stem cells enables the body to increase bone growth in the jaw through angiogenesis to permanently support the dental implant."
Bone regeneration -- in the form of grafting and guided tissue regeneration -- is not new to oral surgery. But using a patient's stem cells to "seed" new dental tissue is. It is part of an emerging field known as regenerative dentistry, and in fact is a driving force behind it. While much of regenerative dentistry has so far been focused on bone and periodontal tissue, some have their sights set on a bigger goal: growing teeth.
At the forefront of this movement is Pamela Yelick, Ph.D., an associate professor of oral and maxillofacial pathology at Tufts University. Her group is using stem cells seeded into biodegradable scaffolds and implanted in an in vivo model to regenerate organized tooth crowns, periodontal tissues, and whole teeth (Journal of Dental Research, August 2008, Vol. 87:8, pp. 745-750).
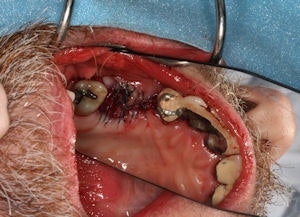 |
| 34-year-old Jason Morgan needs an implant to replace a missing maxillary molar, but without bone regeneration his jaw will not be strong enough to support it. All images courtesy of Dr. Ivan Ho. |
In related work, Takashi Tsuji and colleagues at the Tokyo University of Science reported that, using cells extracted from mouse embryos, they were able to create and transplant a tooth bud into an adult mouse, where it continued to grow into a full-size tooth (Nature Methods, March 2007, Vol. 4:3, pp. 227-230).
More recently, scientists from Nippon Medical School in Tokyo demonstrated that stem cells derived from bone marrow can promote periodontal tissue regeneration (Tissue Engineering Part A, June 2008, Vol. 14:6, pp. 945-953). And researchers from Australia, China, Taiwan, Colombia, Brazil, and Iran will report their latest work in this area at the upcoming International Association of Dental Research meeting in Miami.
Still, when it comes to growing teeth, a number of challenges remain -- especially because the process requires recreating not just the tooth's anatomy but its developmental processes.
"Regenerating a tooth is not an easy task," said Charles Sfeir, D.D.S., Ph.D., director of the Center for Craniofacial Regeneration at the University of Pittsburgh. "The tooth as a structure has multiple tissues, both hard and soft -- cementum, enamel, dentin, pulp, etc. So we are looking at how to regenerate each of them separately. Then we will begin to look at how to put them all together."
Kim Gowey, D.D.S., past president of the American Academy of Implant Dentistry, says that while such work shows great promise, many questions remain.
"Even if you could regenerate a tooth, how would you know it will fit the patient and is in the right spot?" Dr. Gowey said. "You have to be able to differentiate between a bicuspid and a molar and an incisor, and you have to get it to erupt. You would have to implant it in the jaw and get it to regenerate there."
Signaling genes
This is where genetics come in, according to Dr. Sfeir.
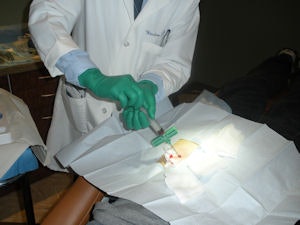 |
| Dr. Ivan Ho harvested and transplanted stem cells from Jason Morgan's hip bone marrow into his jawbone to prompt the growth of new bone in preparation for implant surgery. |
"One of our biggest successes so far is recognizing that when dentin is formed, the proteins being made by the tooth cells have the ability to signal and tell the cells what to do," he said. Understanding genetic signaling is key to being able to develop the specific tissues that comprise a tooth and to growing each specific type of tooth, he added.
"We would all love to say 'grow a molar or grow an incisor,' but I don't think anybody can do this yet at this point. But if we can grow a structure into the bone, and the prosthodontist can put a crown on it, we have achieved a biological implant," Dr. Sfeir said, noting that researchers from Kings College in London, under the direction of Paul Sharpe, Ph.D., a craniofacial biology professor, have already achieved this in mice.
Sharpe and his team demonstrated that stem cells can be prompted to form tooth rudiments when cultured with the appropriate signals. The developing cells showed characteristic markers of tooth development in culture when implanted into mice, according to a presentation Sharpe made at the American Association for the Advancement of Science meeting in 2004.
Having established a company, Odontis, to commercialize this technology, Sharpe is now working to provide proof of principle using human cells and to develop a reproducible methodology for fabricating what they have dubbed the BioTooth -- a tooth bud created from a patient's stem cells that can be implanted into the jaw and grown into a molar, incisor, or bicuspid.
So when might any of this science make its way into the dental office? General consensus is at least five to 10 years. In the meantime, Sharpe told DrBicuspid.com that interest in the BioTooth has already proved so keen that he didn't want to be interviewed for this article.
"I am avoiding doing press interviews since every time something appears, I get swamped with calls from patients," he wrote in an e-mail.
If that is any indication of the commercial potential for bio-teeth, it should be well worth the wait.
In addition to stem cells, a number of methods are being studied for growing bio-teeth; for a comprehensive overview of the various techniques, see Tissue Engineering, Part B: Reviews, 2008, Vol. 14: 3, pp. 307-318.
Copyright © 2009 DrBicuspid.com