Guided surgery company X-Nav Technologies has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its X-Mark patient registration technology.
X-Mark is part of the company's X-Guide dynamic 3D navigation system for guided dental procedures. It enables virtual-based registration of a patient's anatomy and automatically adds that registration to a digital treatment plan.
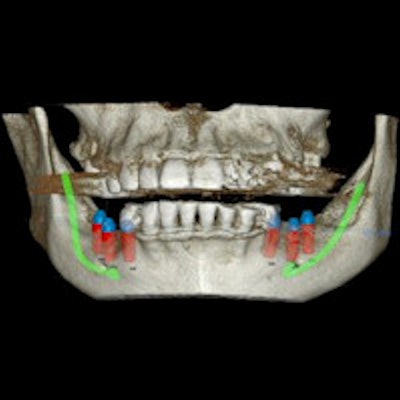
With X-Mark technology, the X-Guide system can match three anatomical marks placed on a patient before surgery with marks in the same three locations on his or her 3D scan. The integration can help dentists accurately and easily perform navigated surgery, according to the company.
Dentists can use the X-Guide system with X-Mark for a range of surgeries, from single tooth replacement to full mouth reconstruction in edentulous patients. X-Guide works with cone-beam 3D scanners to provide live surgical guidance, which X-Nav likened to a GPS for the dental drill.