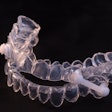
Keystone Industries has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its KeySplint Soft 3D printing resin for night guards and splints.
Recently highlighted at the 2019 International Dental Show (IDS) in Cologne, Germany, KeySplint Soft is a tough yet flexible splint designed to withstand the wear and tear of night teeth grinding. The dental splint resin is resistant to abrasions and fractures and is currently available in two shades: a clear version for exclusive use with 3D printing manufacturer Carbon's 3D printing platform and an open-source violet-tinted version.
KeySplint Soft is one of two materials of its kind to be cleared by the FDA for splint and night guard applications, Keystone noted. The company has validated its resin with several 3D printers, including printers by Carbon, Asiga, and MiiCraft.