Nobio has received an additional 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its Infinix antibacterial composites.
After submitting clinical data showing the composites significantly reduce tooth demineralization, a key part of the caries-formation process, the FDA issued another clearance.
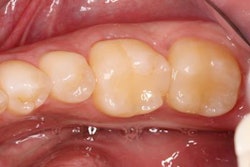
A University of California, San Francisco (UCSF) study assessed Infinix's antibacterial activity and found that mineral loss for teeth adjacent to Infinix composites was two-thirds less than for the standard composite material, according to the company. This supports Infinix's potential for less recurrent decay, longer-lasting restorations, and less natural tooth loss.
Nobio plans to initiate commercial sales of its Infinix product line by the end of 2020. Free starter kits can be reserved on the company's website.