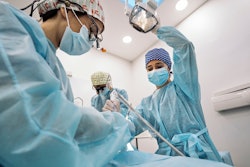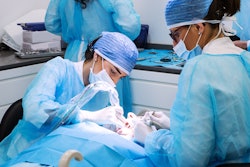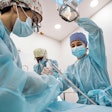
Bone graft materials contaminated with tuberculosis (TB) were used in three dozen dental and medical procedures, causing infections in five people, including one who died, according to the U.S. Centers for Disease Control and Prevention (CDC).
The CDC is warning hospitals and dental offices to identify employees who may have been exposed to the potentially deadly bacterial infection, which affects the lungs and spreads through respiratory droplets from an affected person, during patient surgery or care. The cases appear to be linked to bone grafts from Silver Springs, MD-based Aziyo Biologics, which voluntarily recalled them on July 13, according to the CDC. This is the second time a tuberculosis outbreak has been linked to Aziyo bone grafts.
The CDC recommends that all patients who received Aziyo’s ViBone Moldable or alloOss Plus (lot No. TDS222820) be treated for tuberculosis even if they have no symptoms. Health and dental providers should ensure that patients who received these bone graft materials get evaluated and receive treatment, which should be coordinated with infectious disease and tuberculosis experts, according to the CDC.
“CDC and FDA are working with state and local health departments, hospitals, surgical centers, and dental offices in the affected states to ensure patients are rapidly evaluated and treated, prevent further patient harm, and determine if additional measures can be taken to prevent similar outbreaks in the future,” according to the CDC.
Furthermore, healthcare providers should report adverse patient outcomes to the U.S. Food and Drug Administration's (FDA) MedWatch Adverse Event Reporting program, as well as notify Aziyo, according to the agency.
About the bone grafts
Between February 27 and June 20, shipments of the bone grafts were sent to 13 hospitals and dental offices in California, Louisiana, Michigan, New York, Oregon, Texas, and Virginia. All involved healthcare providers and states have been notified about the bone grafts and all unused units of the products, which have been made from human tissue, have been removed from inventory and will not be used.
Aziyo’s recall notice states that the products were voluntarily pulled after it learned of postsurgical tuberculosis infections in two patients after they were treated with viable bone matrix product from a single donor. Prior to release of the products, samples from this specific lot had tested negative for Mycobacterium tuberculosis by an independent laboratory using a nucleic acid test that is designed to specifically detect the organism that causes tuberculosis, according to a company press release announcing the recall. Additionally, the company suspended shipments of all viable bone matrix products from all donor lots “out of an abundance of caution,” Aziyo said.
“We are taking immediate action to safeguard patients by implementing a full product recall as we work with the CDC to investigate this event,” Dr. Randy Mills, Aziyo’s president and CEO, said in the recall notice. “The people of Aziyo care deeply about the patients we serve and will continue to work with the medical community, patients, and regulatory authorities as we gather additional information.”
Past problems with TB-infected bone grafts
Though it is highly unusual for tuberculosis to be associated with transplanted tissues, this is not the first time that products from Aziyo Biologics have been associated with a tuberculosis outbreak. In the spring of 2021, the company voluntarily recalled its FiberCel viable bone matrix, as it was linked to a large tuberculosis outbreak in at least 87 patients. Eight patients died.
This outbreak was linked to tainted material that came from one single tissue donor, a deceased man. An investigation into the outbreak, which was published in August 2022 in Lancet Infectious Diseases, determined that the donor had “unrecognized risk factors, symptoms, and signs consistent with tuberculosis.”