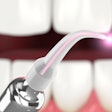
The U.S. FDA has given 510(k) clearance to Biolase Technology to market its handheld iLase diode laser, a "personal laser" designed for dentists and hygienists to perform a range of minimally invasive soft-tissue and hygiene procedures, according to the company. The product, which already had CE Mark approval for sale in Europe, was introduced to the U.S. dental market at the recent Chicago Dental Society Midwinter Meeting.
The iLase includes a diode laser, user interface, battery power, and controls in a single, integrated handpiece with no foot pedals or cords attached. It is about the size of a large pen and has 5 watts of power. It is controlled by a patent-pending finger switch on the handpiece that features Biolase's ComfortPulse control.
The iLase has 10 preset soft-tissue and hygiene procedures, including gingivectomy, troughing for crown procedures, and sulcular debridement, and a procedure for cleaning between gums and teeth for treating periodontal disease.
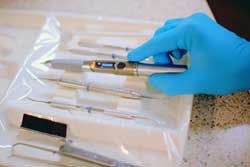 |
| The Biolase iLase diode laser. Image courtesy of Biolase Technology. |
Like the ezlase, the iLase can be used to perform 25 FDA-cleared soft-tissue and hygiene procedures. It can also perform 20-minute teeth whitening and FDA-cleared pain relief functions.
The iLase will begin shipping in April, the company said.
Copyright © 2010 DrBicuspid.com