
One more knight has sallied forth against the biggest dragon in dentistry as a Texas-based start-up released Identafi 3000, a new oral cancer screening device.
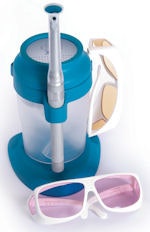 |
| The Identafi comes with a wand and tinted glasses for patient and doctor. Image courtesy of Trimira. |
"Acknowledging the problems with false positives in autofluorescence, we wanted to give the operator additional information," said Andrés Zuluaga, Ph.D., M.S., vice president for research and development for Houston-based Trimira, which launched Identafi at the 2009 Chicago Dental Society Midwinter Meeting.
Trimira developed the device at a cost of $40 million in federal, state, and private funding over the course of 15 years, and it includes venture capitalist T. Boone Pickens among its investors, according to CEO David A. Burns.
If successful, Identafi could prove a bonanza for its investors and a boon for public health. Oral cancer is on the rise and already kills about one person per day. It's much easier to treat if caught earlier.
And several previous attempts at an accurate screening device have failed to garner broad support from experts in the disease. For example, a July 2008 review in the Journal of the American Dental Association (Vol. 139:7, pp. 896-905) concluded that there wasn't enough evidence for the use of toluidine blue (TB), ViziLite (by itself), ViziLite Plus with TBlue, Microlux DL, Orascoptic DK, VELscope, or the OralCDx brush biopsy as screening techniques in "general dental practice settings." Debate on these techniques continues.
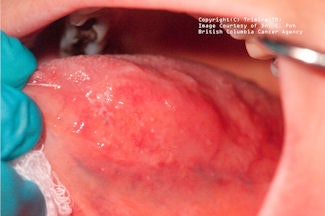 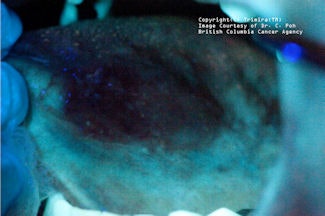 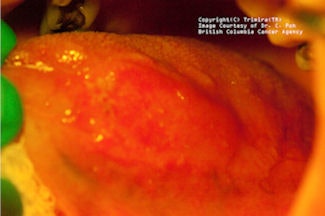 |
| Top, a lesion shown under white light. Middle, this lesion, shown under violet light, appears dark. Bottom, under amber light, the vasculature of the lesion appears. Clinical images courtesy of C. Poh, British Columbia Cancer Agency. |
But whatever their strengths or weaknesses, Identafi differs from these devices because it uses amber as well as white and violet light. It is cordless, handheld, and resembles a dental mirror, with a band that can be rotated to toggle among the three colors of light. It comes with tinted glasses for both the doctor and the patient.
Doctors can use white light for a typical visual examination. After spotting areas of concern, they shine violet light in the mouth. Healthy tissue absorbs this light and emits excitation light (autofluorescence). Unhealthy tissue emits light at a different frequency. The photosensitive glasses block the light from abnormal tissue, making it appear dark.
VELscope also uses autofluorescence to identify cancer, and ViziLite and Microlux work on a similar principle.
But this approach by itself can produce a high rate of false-positive results, Zuluaga said. The problem is that other types of unhealthy tissue can look like cancer. "It turns out that if you bite yourself, you get a similar loss of autofluorescence," he said.
Burns cited the case of a patient who singed his mouth on a Philly cheesesteak, then forgot about the incident. The burned tissue showed up dark in an autofluorescence screening.
What's different about Identafi is the next step. The doctor shines amber light (which actually appears green) on the areas of concern. Because hemoglobin strongly absorbs this light while other substances reflect it, the vasculature of the area is highlighted.
Both healthy tissue and cancer have blood vessels. But healthy tissue grows the vessels in an orderly formation like the roots of a tree, while cancer is more likely to grow vessels in a chaotic, cloudlike formation. If a lesion looks like cancer in both violet and amber light, the patient could be referred to an expert for a biopsy.
Identafi is approved by the FDA as a screening device -- a method of calling attention to patients at high risk -- but not as a diagnostic device.
Identafi has other advantages, too, Zuluaga said. "It's small, it's economical, and it provides results right away. You don't have to send away for something." The device, which sells for $3,000, can reach easily into the back of the mouth, he added.
Down the road, Trimira's parent company, Remicalm, is working on a device that will analyze autofluorescent light to actually diagnose cancer in the mouth and other organs. But that product is a long way from the market, Burns said.
It all sounds good. But the implication that Identafi detects cancer more accurately than competing devices raised the eyebrows of at least one scientist. "What clinical evidence do you have that it does that?" asked Michael Rethman, D.D.S., M.S., the former director of the U.S. Army Institute of Dental Research, who attended the press conference where the device was announced. "I'm not questioning the plausibility," he said. "But as they say, 'Show me the money.' "
Burns responded that a study on the device by researchers from the British Columbia Cancer Agency would be published in about three months, and the University of Texas M. D. Anderson Cancer Center and others are also researching it. "We have run about 125 screenings on this," he said.
Dr. Rethman will be eager to see those results. "I hope they succeed," he said. "We really need a good screening device."
CORRECTION
This article's reference to a study published in the Journal of the American Dental Association was corrected on 3/6/09.
Copyright © 2009 DrBicuspid.com



















