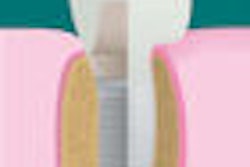
The U.S. Food and Drug Administration (FDA) has released a proposal to reclassify blade-form endosseous dental implants, opening the door for a possible resurgence of this implant technology.
The benefits of blade-form implants "outweigh the risks enough to justify reclassifying the implants from class III, requiring premarket approval, to class II, requiring premarket notification with special controls," the FDA noted in a press release.
Class II medical devices are of "medium risk," while class III is a "high risk" medical device that is also highly regulated.
"Implantologists should know that this [blade implants] is and has always been a viable implant to treat patients," Nick Caplanis, DMD, MS, president of the American Academy of Implant Dentistry, told DrBicuspid.com. "Especially those with certain bone deficiencies that could not otherwise have a root-form dental implant, or for patients that, for one reason or another, can't or don't want to pursue an augmentation bone graft procedure to build the bone back up to accommodate a dental implant or conventional dental implant."
The FDA previously considered reclassifying blade implants in the 1990s but opted not to, stating that "sufficient evidence had not yet been presented to reclassify blade-form endosseous dental implants to class II." As more information has become available, the FDA has modified its position.
The new proposal was drawn up on the agency's own initiative, the organization noted.
"A review of the applicable clinical literature indicates that the device has a high success rate (remaining implanted/not removed) and that few relevant adverse events have been reported in the case of these devices or related devices suggesting that the device has a high long-term safety profile," the proposal states.
The agency plans to apply special controls, including design requirements, mechanical performance testing, corrosion testing under simulated physiological conditions, biocompatibility requirements, sterility testing, testing to ensure device compatibility with magnetic resonance environments, both clinical and patient labeling requirements, and documented clinical experience, including data on adverse events.
A comment period deadline of April 15 is set.
Optimism among old concerns
The change could increase the profile of this type of implant among practitioners and lead to a resurgence in their use, according to Dr. Caplanis.
"Over the last 30 years [blade-form implants] have fallen out of favor for a number of different reasons," he stated. "This might be a way for this implant to make a new splash on the scene of contemporary implant dentistry."
“We welcome the changes that the FDA has proposed.”
American Academy of Implant
Dentistry
The arrival of the Brånemark implant method in the U.S. in the 1980s, buttressed with significant research and high success rates, rapidly changed the type of implants that American practitioners were placing.
"The pendulum swung," Raymond Choi, DDS, an assistant clinical professor in the TMJ/Facial Pain Clinic at the University of Southern California and a private practitioner with a background in minidental implants, told DrBicuspid.com. "Up until that point, implant dentists in America were mainly placing blades and subperiosteals with a very low success rate. When they worked, they worked beautifully."
Questions about achieving osseointegration inevitably entered the discussion about the viability of blade-form implants, he added.
"Their protocol at the time was you placed the implant, you finished the restorative fairly quickly," he said. "And one of the criticisms from the people placing conventional implants was that because of early movements, you'd get fibril integration instead of osseointegration. Therefore, the implant was set for failure in the future."
The FDA also acknowledged health risks associated with the implants, including tissue degeneration, pain, bone and nerve damage, infection, and immune reactions to materials in the devices.
Filling a niche
However, there is an existing niche for blade-form dental implants placed by a skilled practitioner, Dr. Choi emphasized.
"I come across so many situations, especially in the posterior mandible, where we're lacking the bone width for the implant," he said. To move forward, the practitioner must -- often with the help of a surgeon -- do extensive, invasive bone grafting so that there is a wide enough area to receive conventional implants, he added.
"That usually is a procedure declined by patients quite often because of additional surgery, cost, and time involved," Dr. Choi said. "A lot of people who would like to get implants are shut out because of the need to do this grafting."
For those patients, a blade-form implant "could be a tremendous option," he added, since the blade can work in narrow ridges.
Dr. Caplanis agreed. "I think with this implant as part of the arsenal of tools an implant dentist has, that will increase the number of patients that he or she can help," he said.
Those practitioners will need proper training, however. "I never actually placed any blades, but I have watched a master place one in my office on one of my patients, so I know what it entails," Dr. Choi said. "But as soon as this becomes official, if there are learning environments where you can pick up the clinical skills, I think I'm going to get on board right away."
While many practitioners with experience in placing blade-form implants have retired, opportunities to learn their craft could resurface with the arrival of the FDA's decision.
"I think if there's a resurgence of this type of implant, there will be more training for dentists to go through in order to learn how to perfect this technique," Dr. Caplanis said. "Our academy has always been supporters of multimodal implant dentistry, which includes both the blade and the subperiosteal implant. We welcome the changes that the FDA has proposed, and would love to see these types of implants be more universally accepted by our colleagues who don't have the training or understanding of the history of them."