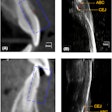
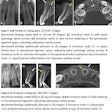
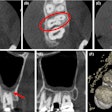
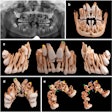
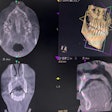
X-Nav Technologies has received 510(k) clearance from the U.S. Food and Drug and Administration (FDA) to expand use of its surgical navigation system for endodontic procedures.
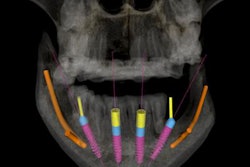
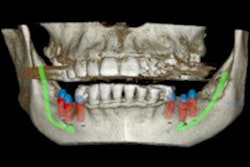
The guided surgery company's X-Guide Dynamic Surgical Navigation System aims to offer more precise, minimally invasive access to structures, specifically calcified canals and apicoectomies of affected teeth, needing endodontic treatment.
Using a patented technology, the system gives clinicians a live 3D view of a patient's anatomy, and a digital handpiece guides drill movements during surgery. Tools within the system aim to make navigation easy to integrate into the endodontic workflow, such as the virtual prep feature for creating and following ideal drill paths and virtual registration, which allows for the use of small, medium, and large field-of-view cone-beam computed tomography images.