Pearl’s artificial intelligence-based dental disease detection software Second Opinion has been granted a Class IIa medical device certification under the European Union Medical Device Regulation (EU MDR).
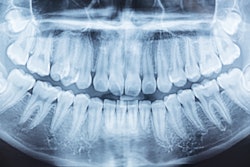
The certification supersedes the EU Medical Devices Directive (MDD) certification, which Second Opinion received in 2021. Second Opinion uses computer vision and machine learning to assist dentists in detecting dental conditions from bitewings and periapical and panoramic radiographs of permanent teeth in patients 12 and older. Dentists in Europe can begin using Second Opinion today.