Inspire Medical Systems, a developer of neurostimulation therapies for the treatment of obstructive sleep apnea (OSA), recently received approval from the U.S. Food and Drug Administration (FDA) to begin a clinical trial of its STAR (stimulation therapy for apnea reduction) implant system.
 |
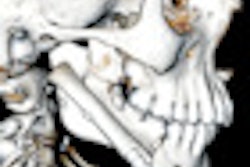
| The STAR II implantable sleep apnea device. Image courtesy of Inspire Medical Systems. |
Medical centers in Germany, Belgium, and the Netherlands are currently approved to implant patients in the STAR trial. Paul Van de Heyning, director of otolaryngology and head and neck surgery at the University of Antwerp, recently implanted the first patient in the STAR trial. The first U.S. implants in the trial are targeted for early 2011.
UAS is an implantable therapy that works with the body's natural physiology to prevent airway obstruction during sleep. While the patient sleeps, Inspire therapy is designed to deliver physiologically timed, mild stimulation to the hypoglossal nerve on each breathing cycle. The stimulation is intended to restore tone to the muscles that control the base of the tongue, preventing the tongue from collapsing and obstructing the airway. Patients control when the therapy is turned on and off via a handheld programmer.
Copyright © 2010 DrBicuspid.com