You're startled by a threatening sound, and your breath quickens. You smash your elbow and pant in pain. Why does your breathing rate increase dramatically when you're hurting or anxious?
As neurobiologists studying how the brain responds to environmental threats and the neural circuitry of emotion, we were curious about the answer to this question ourselves. In our recently published study, we discovered that one particular circuit of the brain in mice underlies this tight connection between pain, anxiety, and breathing. And this discovery may eventually help us develop safer pain killers for humans.
The part of the brain that takes the breath away
One of the most common symptoms of both pain and anxiety disorders is shortness of breath, or hyperventilation. On the other hand, slow, deep breathing can reduce pain and distress. The simplest way to explain this, we reasoned, is the existence of a common pathway in the brain that regulates breathing, pain, and anxiety simultaneously.
So we searched for brain regions previously reported to regulate breathing, pain, and emotion. A small area in the brainstem called the lateral parabrachial nucleus caught our attention. Not only is it part of the breathing regulation center of the brain, it also mediates pain and negative emotions like fear and anxiety.
 A cluster of neurons with highly active opioid receptors, highlighted in neon green, is located in a small area of the mouse brainstem. Salk Institute, CC BY-NC-ND
A cluster of neurons with highly active opioid receptors, highlighted in neon green, is located in a small area of the mouse brainstem. Salk Institute, CC BY-NC-NDSearching through a public database of gene expression patterns, or how genetic material is translated into proteins that let cells function, in the mouse brain, we serendipitously found that one type of opioid receptor called the µ-opioid receptor is highly expressed in parabrachial neurons.
Opioids are chemical compounds that decrease pain and promote positive emotions. But they can also slow breathing rates to dangerously low levels, or stop breathing altogether. This repression of breathing is the main reason opioid overdoses cause death.
Previous studies have shown that the effects opioids have on the body are mainly mediated by µ-opioid receptors. We therefore focused our investigation on how pain and breathing interact with each other in neurons that express these receptors.
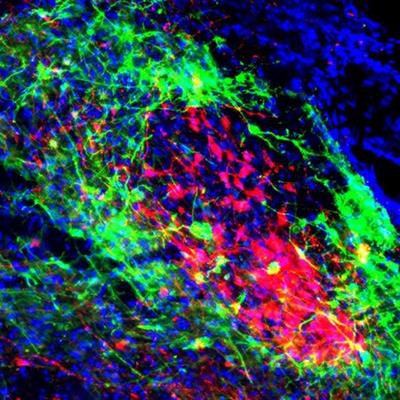
We labeled the neurons projecting to the breathing and pain centers with multicolored fluorescent proteins. In doing this, we were able to identify two subsets of neurons that express µ-opioid receptors. These neurons were arranged in a core-shell shape, where one subset of neurons are wrapped around the other subset. The outer shell neurons regulate breathing by sending their axons -- the long part of the neuron that transmits electrical signals -- to the part of the brainstem that controls breathing. The inner core neurons, on the other hand, mediate pain and anxiety by sending their axons to the brain's pain and emotion center, the amygdala.
 Shell neurons, colored in green, project to the brain's breathing center in the brainstem. Core neurons, colored red, project to the pain/emotion center called the amygdala. Salk Institute, CC BY-NC-ND
Shell neurons, colored in green, project to the brain's breathing center in the brainstem. Core neurons, colored red, project to the pain/emotion center called the amygdala. Salk Institute, CC BY-NC-NDWhat we found even more interesting was that the core and shell neurons interact with each other -- activating one subset of neurons will send a signal to the other. This interconnected loop can explain why breathing, pain, and anxiety are often regulated simultaneously and influence one another.
If a similar loop exists in humans, this may also explain why your breath shortens when you are afraid or in pain.
Why is breathing linked to anxiety and pain?
When animals encounter a harmful or threatening situation, their oxygen levels rapidly increase to help them escape from danger. This might be why breathing and pain are tightly coupled.
Previous studies have shown that neurons in the parabrachial nucleus are critically important in inducing shortness of breath during conditions like hypercapnia, where there is too much carbon dioxide in the blood from breathing too shallowly, and hypoxia, where the body is deprived of oxygen. Telling the body to increase its breathing rate helps decrease carbon dioxide levels and replenish vital oxygen stores.
Going further, our results suggest that these neurons serve as a central alarm system in the brain. When these neurons fire, they trigger behavioral and physiological responses that help animals cope with external threats, like predators, and internal threats, like low oxygen levels.
Designing safer drugs to alleviate pain
Our findings could lead to the development of safer pain-relieving drugs that don't dangerously repress breathing.
From 1999 to 2018, opioid overdoses killed 4.5 million people in the U.S. alone. Directly causing these deaths are the dangerously low breathing rates, or opioid-induced respiratory depression, that are a side effect of these drugs.
Neurons in the parabrachial nucleus affect breathing and pain in different ways and harbor numerous receptors that could be used as drug targets. Our research team is now performing genetic analyses of these neurons in mice to identify receptors that can specifically turn up or turn down these pain and breathing pathways.
If similar neurons are found in humans, we would move one step closer to developing safer pain-relieving drugs and potentially reducing opioid overdose deaths.
Article reprinted under Creative Commons license from The Conversation.
Sung Han is an assistant professor at the Salk Institute for Biological Studies and an assistant adjunct professor of neurobiology at the University of California, San Diego (UCSD). Shijia Liu is a doctoral candidate in neurobiology at the Salk Institute for Biological Studies, UCSD.
The comments and observations expressed herein do not necessarily reflect the opinions of DrBicuspid.com, nor should they be construed as an endorsement or admonishment of any particular idea, vendor, or organization.